Dewar Flask Explosion . examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Wondering how much oxygen would get. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. looks like that is a vacuum flask dewar. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. how to calculate percentage oxygen depletion incase of dewar failure.
from www.fortunesci.co.th
Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. how to calculate percentage oxygen depletion incase of dewar failure. looks like that is a vacuum flask dewar. Wondering how much oxygen would get. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture.
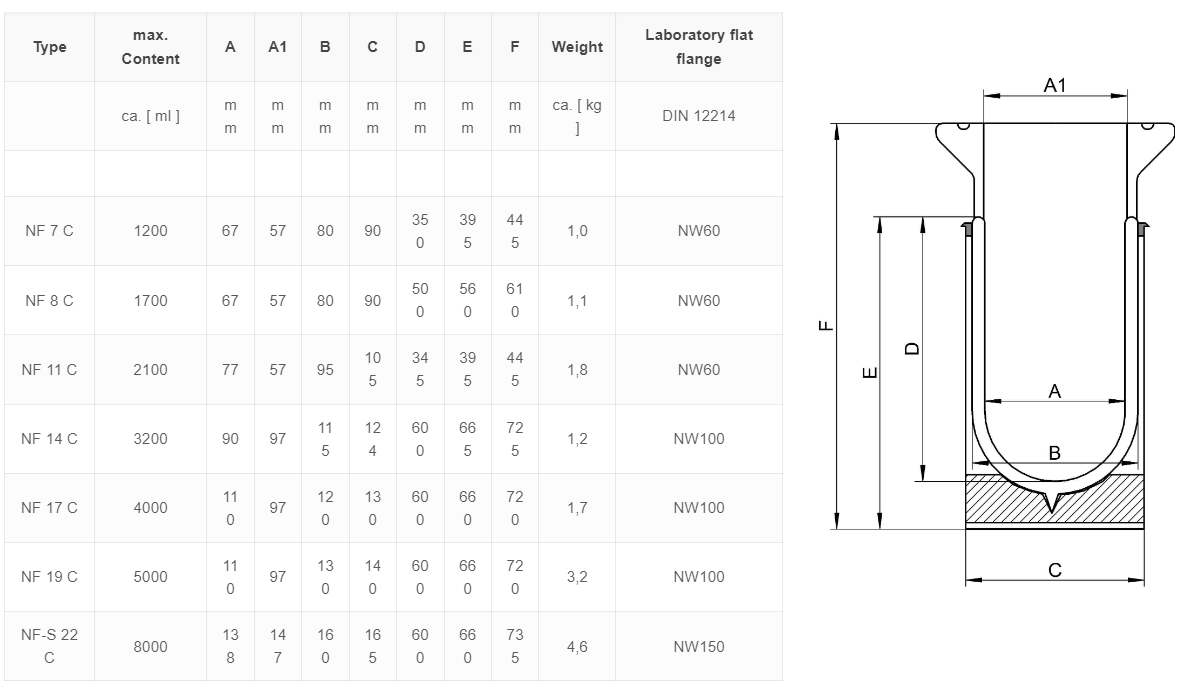
Dewar flasks with melt on Laboratory flat flange
Dewar Flask Explosion Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. looks like that is a vacuum flask dewar. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. how to calculate percentage oxygen depletion incase of dewar failure. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. Wondering how much oxygen would get.
From mylabmart.com
Dewar Carrying Flask, KGW Isotherm Germany AB LAB MART Dewar Flask Explosion Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask.. Dewar Flask Explosion.
From www.scribd.com
Calibration of Dewar Flask PDF Dewar Flask Explosion looks like that is a vacuum flask dewar. how to calculate percentage oxygen depletion incase of dewar failure. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Wondering how much oxygen would get. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. created in 1892. Dewar Flask Explosion.
From coleparmer.com
Low Form Dewar Flasks glass and aluminum 275 mL from ColeParmer Dewar Flask Explosion how to calculate percentage oxygen depletion incase of dewar failure. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. looks like. Dewar Flask Explosion.
From www.alamy.com
James Dewar's ( 18421923) inventor of the vacuum flask. His old flasks Dewar Flask Explosion looks like that is a vacuum flask dewar. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Wondering how much oxygen would get. how to calculate percentage oxygen depletion incase of dewar failure. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of. Dewar Flask Explosion.
From www.pamalyne.com
Lab Grade Dewars, กระติ๊กเก็บความเย็น, กระติ๊กใส่สารไนโตรเจนเหลว, Dewar Dewar Flask Explosion looks like that is a vacuum flask dewar. Wondering how much oxygen would get. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. invented by sir james dewar in 1892, the vacuum flask consists of two flasks,. Dewar Flask Explosion.
From www.day-impex.co.uk
Dilvac Dewar Flasks DayImpex Ltd Dewar Flask Explosion created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. Wondering how much oxygen would get. Unfortunately, as opposed to a standard dewar, these. Dewar Flask Explosion.
From www.day-impex.co.uk
Dilvac Dewar Flasks DayImpex Ltd Dewar Flask Explosion invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. looks like that is a vacuum flask dewar. Wondering how much oxygen would get. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. how to calculate percentage oxygen. Dewar Flask Explosion.
From www.fortunesci.co.th
Dewar flasks with melt on Laboratory flat flange Dewar Flask Explosion created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. Wondering how much oxygen would get. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. looks like that is a vacuum flask dewar. invented by sir james dewar in 1892,. Dewar Flask Explosion.
From jdmlabsolutions.com
Stainless Steel Dewar Flasks JDM Lab Solutions Dewar Flask Explosion Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck.. Dewar Flask Explosion.
From www.researchgate.net
Dewar flasks used in the present study, from left to right GSS 2000 Dewar Flask Explosion looks like that is a vacuum flask dewar. how to calculate percentage oxygen depletion incase of dewar failure. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. invented by sir james dewar in 1892, the vacuum. Dewar Flask Explosion.
From www.pinterest.co.uk
Dewar or vacuum flask fully labelled diagram with editable layers Dewar Flask Explosion how to calculate percentage oxygen depletion incase of dewar failure. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Unfortunately, as opposed to a standard dewar, these are at risk of. Dewar Flask Explosion.
From www.thelabstore.co.uk
KGW Isotherm Dewar Flasks with Glass Screw Thread Dewar Flask Explosion how to calculate percentage oxygen depletion incase of dewar failure. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. examples of compounds that oxidize, decompose, or explode under. Dewar Flask Explosion.
From www.dreamstime.com
Vacuum Flask Or Dewar Flask Stock Vector Illustration of physics Dewar Flask Explosion Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. how to calculate percentage oxygen depletion incase of dewar failure. Wondering how much oxygen would get. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. created in 1892 to hold liquefied gases at extremely low temperatures, this. Dewar Flask Explosion.
From www.indiamart.com
INOX Aluminum Spherical Dewar Flask, For Semen Preservation, Capacity Dewar Flask Explosion invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask.. Dewar Flask Explosion.
From www.amazon.ca
Amazon.ca Dewar Flasks Industrial & Scientific Dewar Flask Explosion how to calculate percentage oxygen depletion incase of dewar failure. Wondering how much oxygen would get. examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. looks like that is a vacuum flask dewar. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within. Dewar Flask Explosion.
From www.dswgascylinder.com
Cryogenic dewar flasks, cryo dewar DSW Global supplier Dewar Flask Explosion examples of compounds that oxidize, decompose, or explode under the influence of oxygen or moisture. Wondering how much oxygen would get. invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. created in 1892 to hold liquefied gases at extremely low temperatures, this. Dewar Flask Explosion.
From www.thelabstore.co.uk
KGW Isotherm Aluminium Brushed Spherical Dewar Flasks Dewar Flask Explosion invented by sir james dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. how to calculate percentage oxygen depletion incase of dewar failure. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos flask. looks like. Dewar Flask Explosion.
From jdmlabsolutions.com
Stainless Steel Dewar Flasks JDM Lab Solutions Dewar Flask Explosion how to calculate percentage oxygen depletion incase of dewar failure. looks like that is a vacuum flask dewar. Unfortunately, as opposed to a standard dewar, these are at risk of spontaneously imploding. Wondering how much oxygen would get. created in 1892 to hold liquefied gases at extremely low temperatures, this object is the forerunner of the thermos. Dewar Flask Explosion.